Page 98 - Remedial Andrology
P. 98
Penile traction therapy
In men with PD, potential mechanisms for disease modification with penile traction therapy (PTT) have been
described, including collagen remodelling via decreased myofibroblast activity and matrix metalloproteinase
up-regulation [1074, 1075].
The stated clinical goals of PTT are to non-surgically reduce curvature, enhance girth, and recover lost length,
which are attractive to patients with PD. However, clinical evidence is limited due to the small number of
patients included (267 in total), the heterogeneity in the study designs, and the non-standardised inclusion and
exclusion criteria which make it impossible to draw any definitive conclusions about this therapy [1076-1080].
Most of the included patients will need further treatment to ameliorate their curvature for satisfactory sexual
intercourse. Moreover, the effect of PTT in patients with calcified plaques, hourglass or hinge deformities which
are, theoretically, less likely to respond to PTT has not been systematically studied. In addition, the treatment
can result in discomfort and be inconvenient due to use of the device for an extended period (2-8 hours daily),
but has been shown to be tolerated by highly motivated patients. There were no serious adverse effects,
including skin changes, ulcerations, hypo-aesthesia or diminished rigidity [1078, 1081].
In conclusion, PTT seems to be effective and safe for patients with PD, but there is still lack of evidence to give
any definitive recommendation in terms of monotherapy for PD.
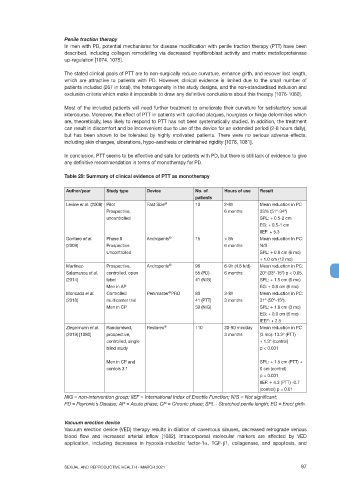
Table 29: Summary of clinical evidence of PTT as monotherapy
Author/year Study type Device No. of Hours of use Result
patients
Levine et al. (2008) Pilot Fast Size ® 10 2-8h Mean reduction in PC
Prospective, 6 months 33% (51º-34º)
uncontrolled SPL: + 0.5-2 cm
EG: + 0.5-1 cm
IIEF: + 5.3
Gontero et al. Phase II Andropenis ® 15 > 5h Mean reduction in PC:
(2009) Prospective 6 months N/S
Uncontrolled SPL: + 0.8 cm (6 mo)
+ 1.0 cm (12 mo)
Martinez- Prospective, Andropenis ® 96 6-9h (4.6 h/d) Mean reduction in PC:
Salamanca et al. controlled, open 55 (PD) 6 months 20º (33º-15º) p < 0.05.
(2014) label 41 (NIG) SPL: + 1.5 cm (6 mo)
Men in AP EG: + 0.9 cm (6 mo)
®
Moncada el al. Controlled Penimaster PRO 80 3-8h Mean reduction in PC:
(2018) multicenter trial 41 (PTT) 3 months 31º (50º-15º).
Men in CP 39 (NIG) SPL: + 1.8 cm (3 mo)
EG: + 0.9 cm (6 mo)
IEEF: + 2.5
Ziegelmann et al. Randomised, Restorex ® 110 30-90 min/day Mean reduction in PC
(2019) [1080] prospective, 3 months (3 mo): 13.3º (PTT)
controlled, single + 1.3º (control)
blind study p < 0.001
Men in CP and SPL: + 1.5 cm (PTT) +
contols 3:1 0 cm (control)
p < 0.001
IIEF: + 4.3 (PTT) -0.7
(control) p = 0.01
NIG = non-intervention group; IIEF = International Index of Erectile Function; N/S = Not significant;
PD = Peyronie´s Disease; AP = Acute phase; CP = Chronic phase; SPL - Stretched penile length; EG = Erect girth.
Vacuum erection device
Vacuum erection device (VED) therapy results in dilation of cavernous sinuses, decreased retrograde venous
blood flow and increased arterial inflow [1082]. Intracorporeal molecular markers are affected by VED
application, including decreases in hypoxia-inducible factor-1α, TGF-β1, collagenase, and apoptosis, and
SEXUAL AND REPRODUCTIVE HEALTH - MARCH 2021 97