Page 95 - Remedial Andrology
P. 95
The original treatment protocol in all studies consists of two injections of 0.58 mg of CCH 24-72 hours apart
every 6 weeks for up to four cycles. Data from IMPRESS (Investigation for Maximal Peyronie´s Reduction
Efficacy and Safety Studies) II and II studies [976], as well as post approval trials [1041], which demonstrated
the efficacy and safety of this treatment, are summarised in Table 27.
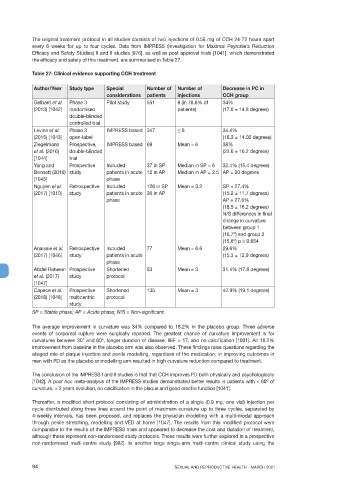
Table 27: Clinical evidence supporting CCH treatment
Author/Year Study type Special Number of Number of Decrease in PC in
considerations patients injections CCH group
Gelbard et al. Phase 3 Pilot study 551 8 (in 78.8% of 34%
(2013) [1042] randomised patients) (17.0 ± 14.8 degrees)
double-blinded
controlled trial
Levine et al. Phase 3 IMPRESS based 347 < 8 34.4%
(2015) [1043] open-label (18.3 ± 14.02 degrees)
Ziegelmann Prospective, IMPRESS based 69 Mean = 6 38%
et al. (2016) double-blinded (22.6 ± 16.2 degrees)
[1044] trial
Yang and Prospective Included 37 in SP Median in SP = 6 32.4% (15.4 degrees)
Bennett (2016) study patients in acute 12 in AP Median in AP = 2.5 AP = 20 degrees
[1045] phase
Nguyen el al. Retrospective Included 126 in SP Mean = 3.2 SP = 27,4%
(2017) [1010] study patients in acute 36 in AP (15.2 ± 11.7 degrees)
phase AP = 27,6%
(18.5 ± 16.2 degrees)
N/S differences in final
change in curvature
between group 1
(16.7º) and group 2
(15.6º) p = 0.654
Anaissie et al. Retrospective Included 77 Mean = 6.6 29.6%
(2017) [1046] study patients in acute (15.3 ± 12.9 degrees)
phase
Abdel Raheem Prospective Shortened 53 Mean = 3 31.4% (17.6 degrees)
et al. (2017) study protocol
[1047]
Capece et al. Prospective Shortened 135 Mean = 3 42.9% (19.1 degrees)
(2018) [1048] multicentric protocol
study
SP = Stable phase; AP = Acute phase; N/S = Non-significant.
The average improvement in curvature was 34% compared to 18.2% in the placebo group. Three adverse
events of corporeal rupture were surgically repaired. The greatest chance of curvature improvement is for
curvatures between 30° and 60°, longer duration of disease, IIEF > 17, and no calcification [1001]. An 18.2%
improvement from baseline in the placebo arm was also observed. These findings raise questions regarding the
alleged role of plaque injection and penile modelling, regardless of the medication, in improving outcomes in
men with PD as the placebo or modelling arm resulted in high curvature reduction compared to treatment.
The conclusion of the IMPRESS I and II studies is that that CCH improves PD both physically and psychologically
o
[1042]. A post hoc meta-analysis of the IMPRESS studies demonstrated better results in patients with < 60 of
curvature, > 2 years evolution, no calcification in the plaque and good erectile function [1041].
Thereafter, a modified short protocol consisting of administration of a single (0.9 mg, one vial) injection per
cycle distributed along three lines around the point of maximum curvature up to three cycles, separated by
4-weekly intervals, has been proposed, and replaces the physician modelling with a multi-modal approach
through penile stretching, modelling and VED at home [1047]. The results from this modified protocol were
comparable to the results of the IMPRESS trials and appeared to decrease the cost and duration of treatment,
although these represent non-randomised study protocols. These results were further explored in a prospective
non-randomised multi-centre study [982]. In another large single-arm multi-centre clinical study using the
94 SEXUAL AND REPRODUCTIVE HEALTH - MARCH 2021