Page 33 - Remedial Andrology
P. 33
consideration, particularly in the management of functional hypogonadism. Testosterone therapy may be
beneficial for hypogonadal men with low or moderate fracture risk [96]; therefore, dual energy X-ray
absorptiometry (DEXA) bone scan may also be considered at baseline and 18-24 months following
testosterone therapy, particularly in patients with more severe hypogonadism [96].
Digital rectal examination may detect prostate abnormalities that can be present even in men with
normal PSA values. Hence, DRE is mandatory in all men at baseline and during testosterone therapy.
The decision to stop testosterone therapy or to perform prostate biopsy due to PSA increase or
prostate abnormalities should be based on local PCa guidelines. There is a large consensus that any increase
of haematocrit > 54% during testosterone therapy requires therapy withdrawal and phlebotomy to avoid
potential adverse effects including venous-thromboembolism and CVD, especially in high-risk individuals. In
patients with lower risk of relevant clinical sequelae, the situation can be alternatively managed by reducing
testosterone dose and switching formulation along with venesection. A positive family history of venous-
thromboembolism should be carefully investigated and the patient counselled with regard to testosterone
therapy to avoid/prevent thrombophilia-hypofibrinolysis [76]. Finally, caution should be exercised in men with
pre-existing CVD or at higher risk of CVD.
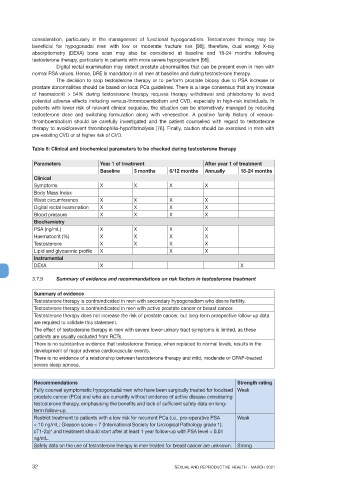
Table 6: Clinical and biochemical parameters to be checked during testosterone therapy
Parameters Year 1 of treatment After year 1 of treatment
Baseline 3 months 6/12 months Annually 18-24 months
Clinical
Symptoms X X X X
Body Mass Index
Waist circumference X X X X
Digital rectal examination X X X X
Blood pressure X X X X
Biochemistry
PSA (ng/mL) X X X X
Haematocrit (%) X X X X
Testosterone X X X X
Lipid and glycaemic profile X X X
Instrumental
DEXA X X
3.7.9 Summary of evidence and recommendations on risk factors in testosterone treatment
Summary of evidence
Testosterone therapy is contraindicated in men with secondary hypogonadism who desire fertility.
Testosterone therapy is contraindicated in men with active prostate cancer or breast cancer.
Testosterone therapy does not increase the risk of prostate cancer, but long-term prospective follow-up data
are required to validate this statement.
The effect of testosterone therapy in men with severe lower-urinary tract symptoms is limited, as these
patients are usually excluded from RCTs.
There is no substantive evidence that testosterone therapy, when replaced to normal levels, results in the
development of major adverse cardiovascular events.
There is no evidence of a relationship between testosterone therapy and mild, moderate or CPAP-treated
severe sleep apnoea.
Recommendations Strength rating
Fully counsel symptomatic hypogonadal men who have been surgically treated for localised Weak
prostate cancer (PCa) and who are currently without evidence of active disease considering
testosterone therapy, emphasising the benefits and lack of sufficient safety data on long-
term follow-up.
Restrict treatment to patients with a low risk for recurrent PCa (i.e., pre-operative PSA Weak
< 10 ng/mL; Gleason score < 7 (International Society for Urological Pathology grade 1);
cT1-2a)* and treatment should start after at least 1 year follow-up with PSA level < 0.01
ng/mL.
Safety data on the use of testosterone therapy in men treated for breast cancer are unknown. Strong
32 SEXUAL AND REPRODUCTIVE HEALTH - MARCH 2021